Research
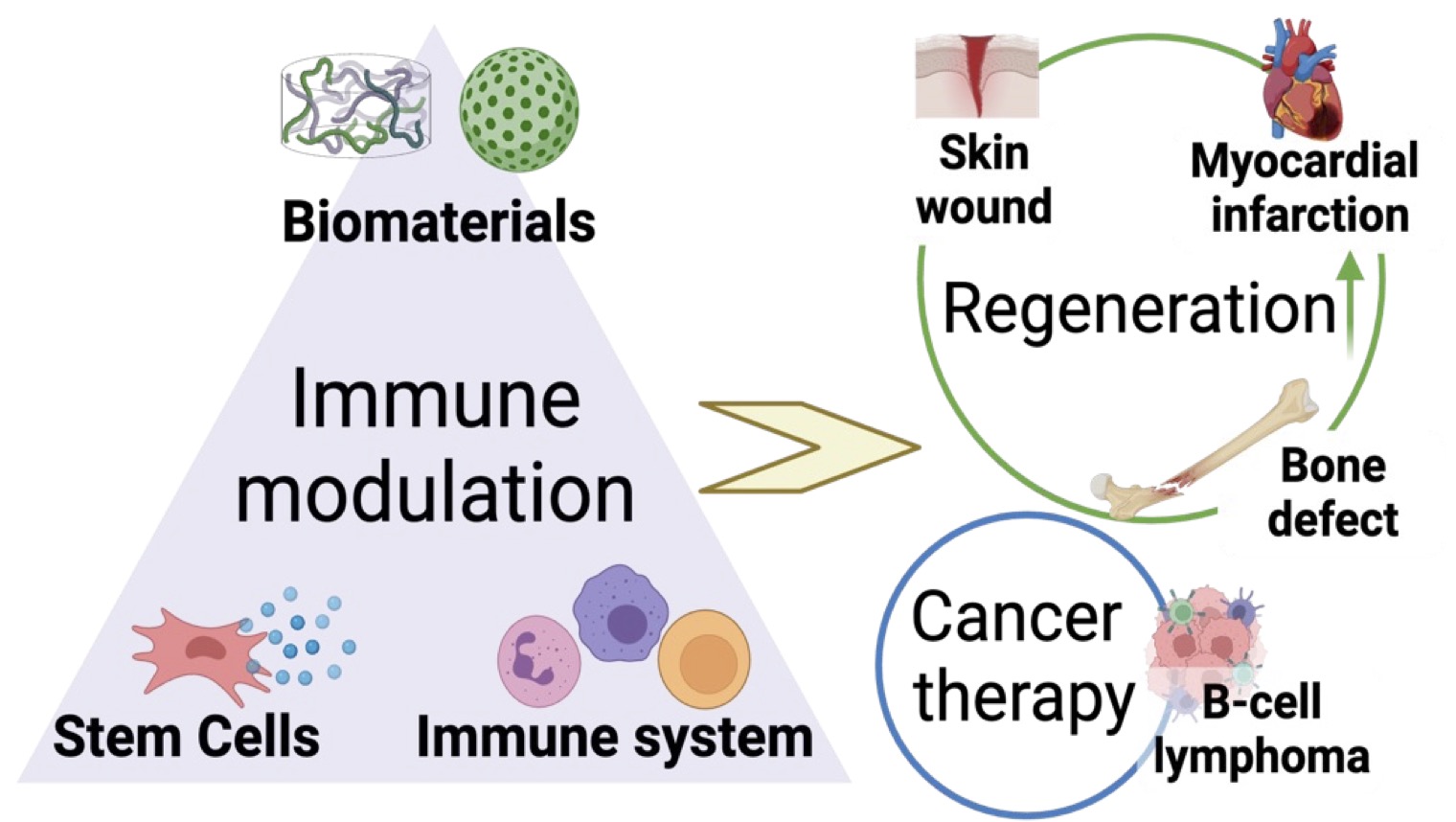
Our group is dedicated to uncovering immune
mechanisms in various disease conditions and
innovating biomaterial-based immunotherapy for
tissue injury, cancer, and aging-induced tissue
degeneration.
Toward this goal, we focus on two
main research directions:
(1) We design biomaterials
with tunable biophysical and biochemical cues to
directly modulate immune activity and promote
tissue regeneration.
(2) We develop humanized in
vitro disease models for mechanistic discovery of
immune crosstalk and high-throughput drug
screening.
By integrating principles of immunology,
materials science, and stem cell biology, we aim to
uncover the underlying mechanisms of immune
dysfunction and leverage this knowledge to
develop next-generation immunotherapies.
Theme 1: Immunomodulatory hydrogel scaffold for bone regeneration
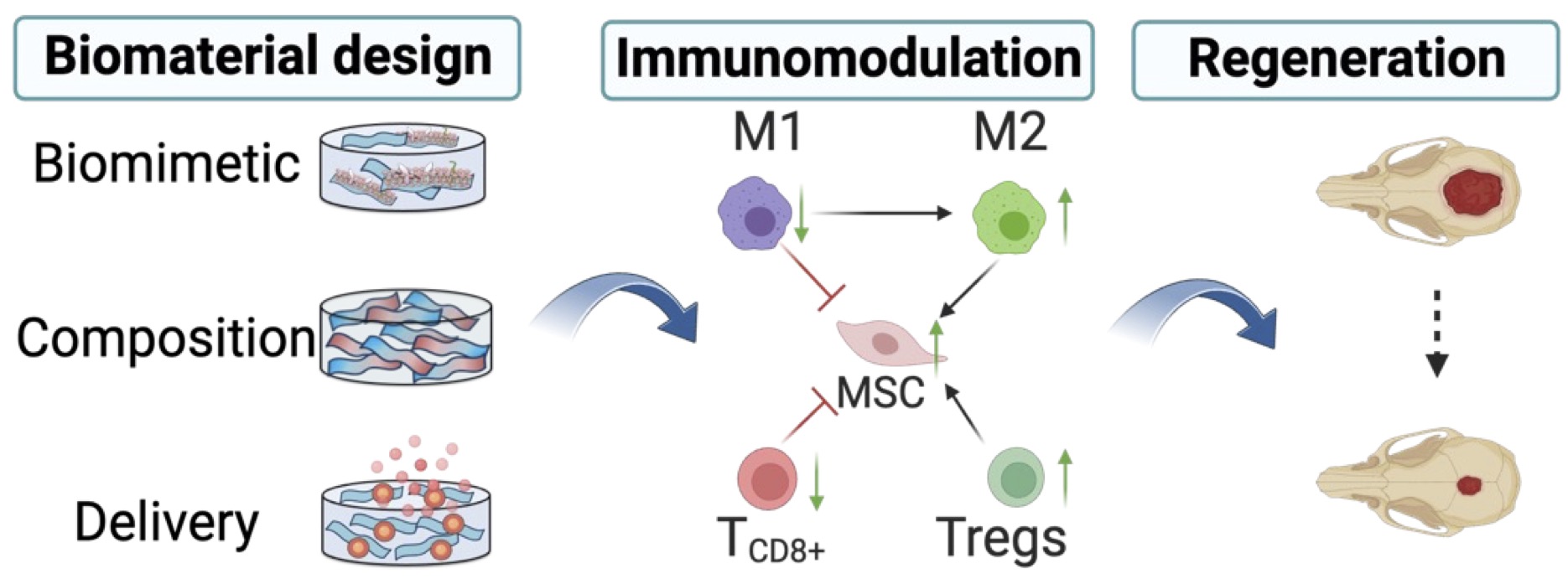
Large bone defects do not heal spontaneously and remain a significant clinical challenge. Immunomodulatory approaches have emerged as a promising new strategy in tissue engineering by harnessing and directing the host immune response to promote tissue regeneration. To address this, we designed an immunomodulatory macroporous hydrogel material for the regeneration of critical-sized bone defects. Our platform integrates innovative strategies including biomimetic material design, tuning of scaffold composition, and controlled delivery of immunomodulatory cues to orchestrate a regenerative immune environment.
Su et al., Advanced Materials, 2023
1.1 Cell membrane coating technology
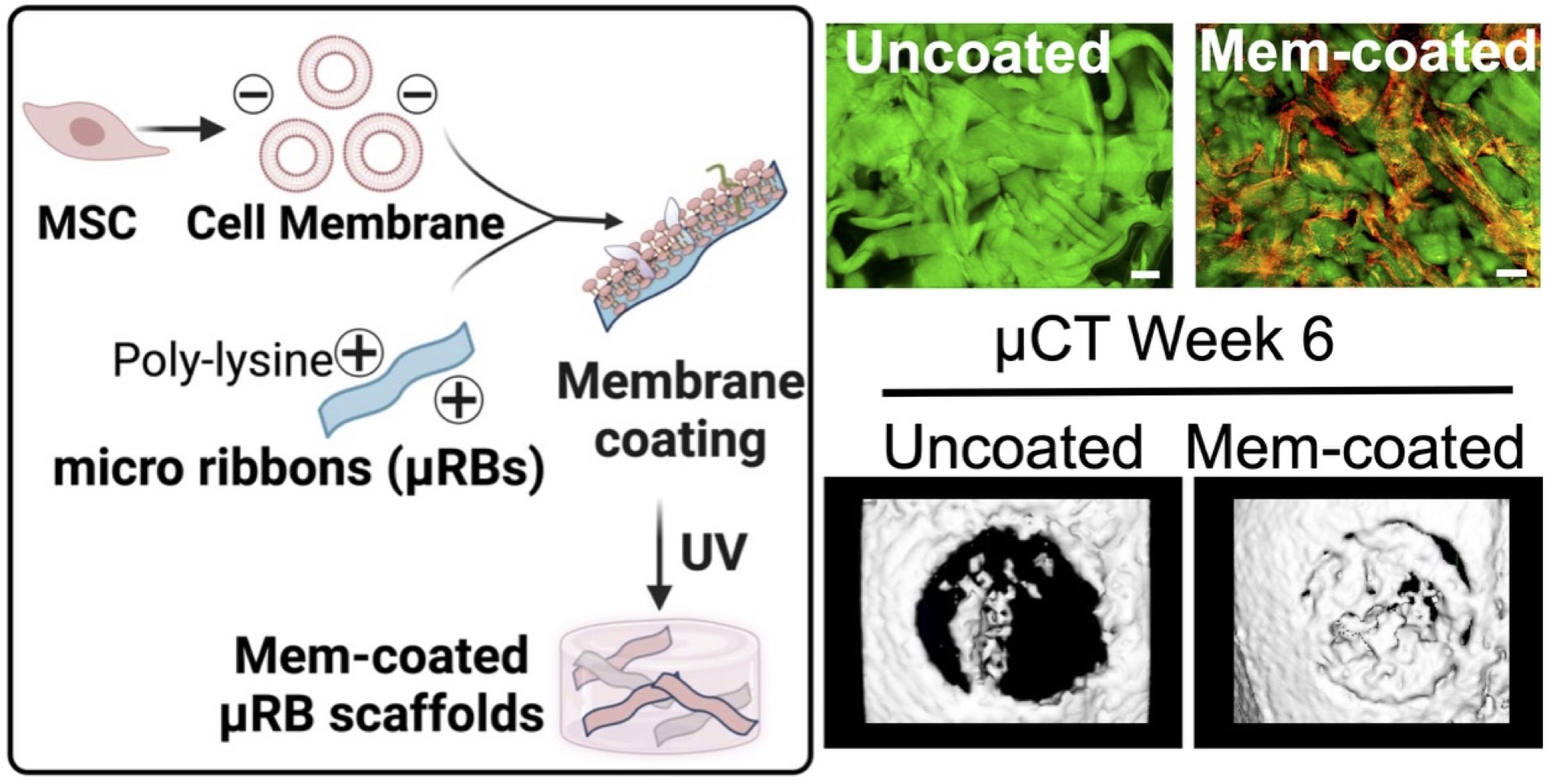
Cell membrane (CellMem) coating offers distinct advantages in biomimicry by leveraging the full repertoire of natural membrane-bound ligands. We pioneered the integration of stem cell-derived membranes onto macroporous hydrogel scaffolds to achieve localized immunomodulation. These CellMem-coated scaffolds modulated both macrophage and T cell responses, resulting in complete regeneration of critical-sized craniofacial bone defects. Our ongoing work further explores cell membranes derived from various cell types and their synergistic effects with cell-secreted vesicles to develop therapies for tissue injury in aging and cancer treatment.
Su et al., npj regenerative medicine, 2025
1.2 Modulating immune-stem cell crosstalk by tuning material composition
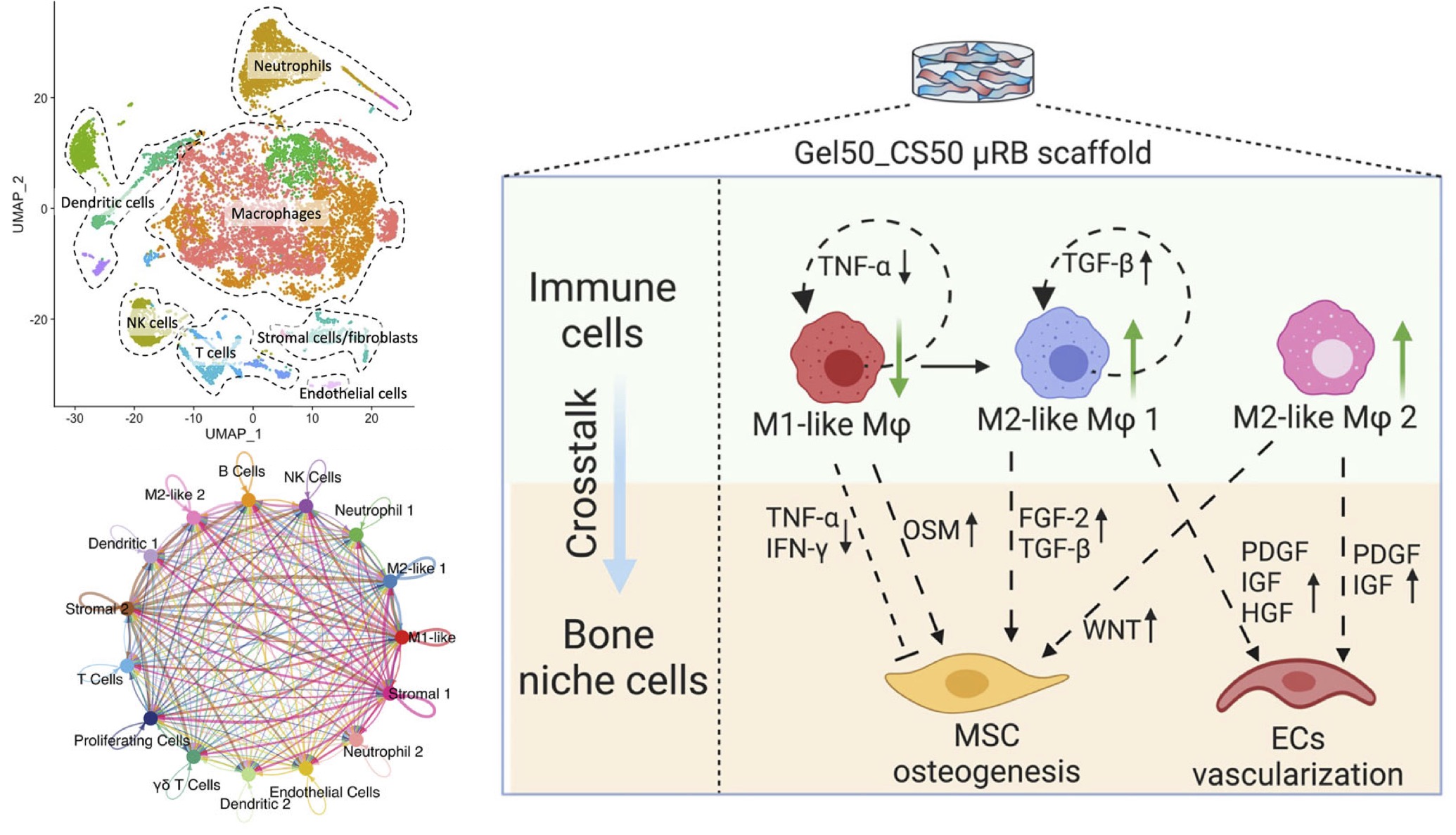
We developed a macroporous scaffold with tunable ratios of gelatin (Gel) and chondroitin sulfate (CS), enabling rapid endogenous bone regeneration in a critical-sized defect model, without the need for exogenous growth factors or transplanted cells. We further harness single-cell sequencing and computational profiling to uncover the mechanisms of immune responses within the bone injury niche in response to varying material composition. This research has identified novel regenerative immune cell populations and revealed unique crosstalk patterns orchestrated by implanted scaffolds.
Theme 2: Engineer immunomodulatory materials with stem cell secretome for skin wound healing
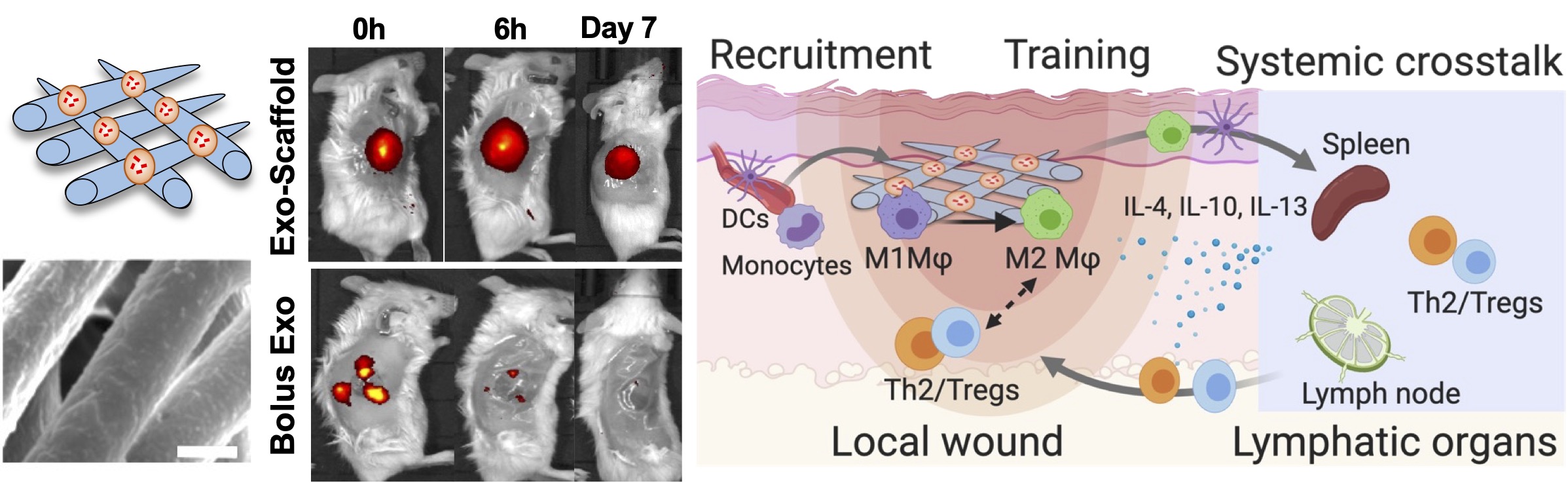
Su et al., Science Advances, 2021
2.1 Exosome-laden scaffold as an immune “training camp.”
Mesenchymal stem cells (MSCs)-secreted exosomes exhibit equivalent immunomodulatory capabilities as live cells, but there remain challenges in the limited retention of MSC exosomes in the injury niche. To address this issue, we developed exosome-functionalized fibrous scaffolds that exhibited prolonged exosome retention in the skin wound area. Inspired by the principles of cancer vaccine development, where scaffolds served as a “training camp” for immune systems to fight against cancer, this innovative design synergized fibrous scaffolds with MSC exosomes, enabling the recruitment and targeted training of the immune system toward regenerative states.
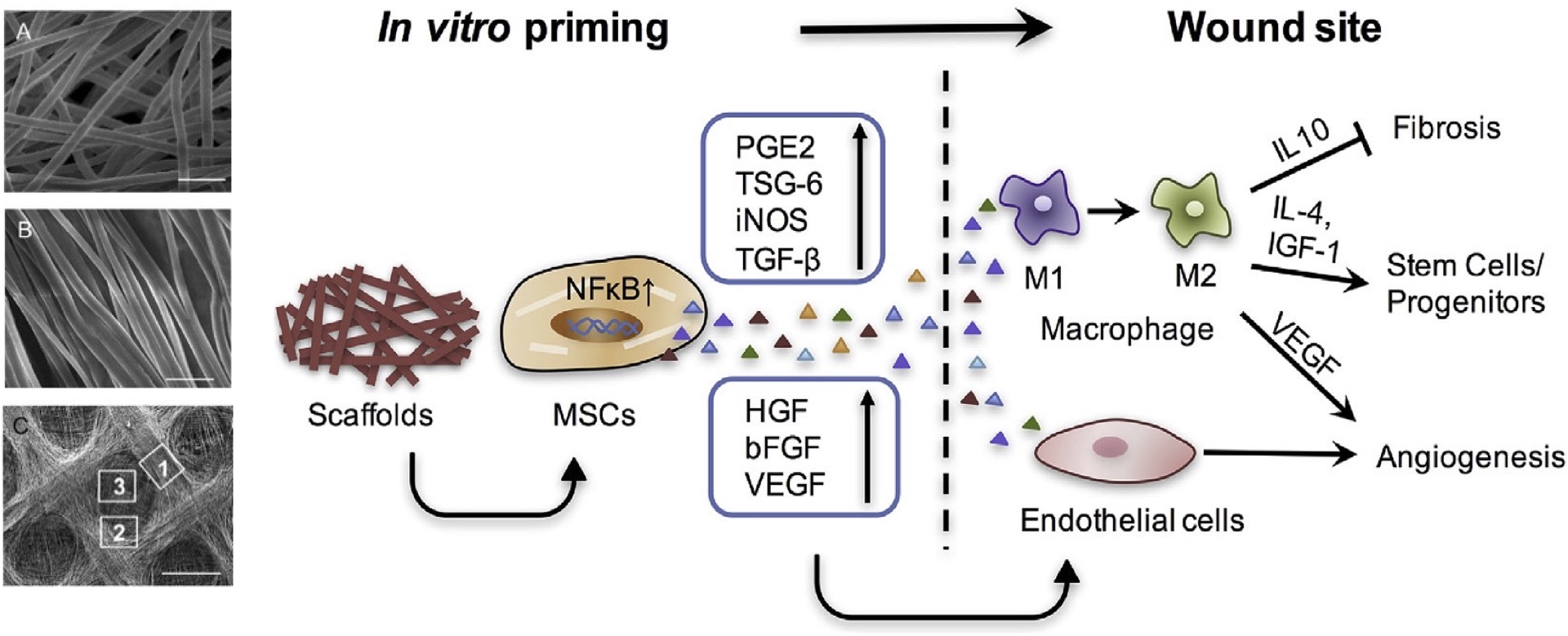
Su et al., Biomaterials 2017
2.2 Harness scaffold geometry to prime stem cell immunomodulatory paracrine function
Given the therapeutic MSC secretome in immunomodulation and angiogenesis, we explored how to improve their therapeutic secretome through cell-material interaction. We engineered topographical patterns of fibrous scaffolds, which increased anti-inflammatory and angiogenic factor release from MSCs, leading to accelerated healing in the skin wound. This study provided the evidence of using scaffold topography to potentiate MSC paracrine function.